

Larger the disorder or randomness of a system, the higher is the value of entropy. Unit of Entropy is Joule per Kelvin per mole ( JK -1 mol -1)Įntropy is a measure of the degree of randomness or disorder of a system. Unit of Entropy : Entropy Change is expressed in calorie per Kelvin per mole( Cal K -1 mol -1) The Calorie per Kelvin ( Cal K -1) is called the entropy unit (e.u). Where q rev = the amount of heat absorbed by the system in a reversible process at a temp T֯ K. ΔS 0 reaction = Σ S 0( products)-Σ S 0 ( reactants )Ĭhange in Entropy of a system is defined as the ratio of heat (q) absorbed by the system isothermally and reversibly to the temperature ( T K) at which heat is absorbed.

When the reactants and the products are at 298 K and 1 atm. The change in entropy during a process when a system undergoes a change from one state to another state, is represented as ΔSįor Chemical reaction, ΔS reaction = Σ S( products)-Σ S ( reactants ) When the state of a system changes, the entropy also changes. Greater the disorder or randomness in a system, the higher will be its entropy.Įntropy is expressed by the symbol S and like internal energy and enthalpy ,entropy is also a state function and so the change in entropy depend only upon the initial and final states of the system.
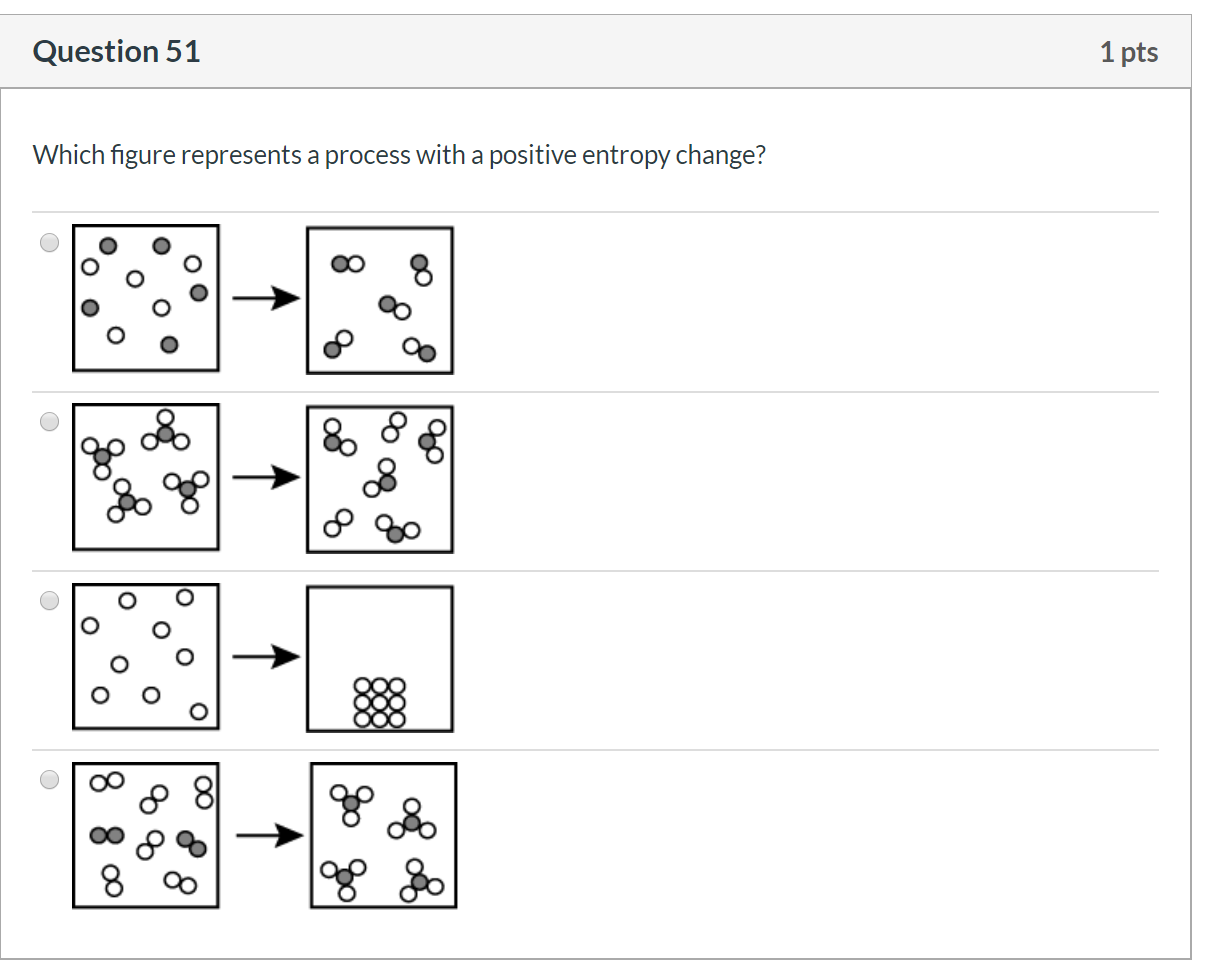
Entropy may be defined as the property of a system which measures the degree of disorder or randomness in a system. Definition of Entropy – The degree of randomness in a system is expressed by a property which is expressed by a property known as entropy.


 0 kommentar(er)
0 kommentar(er)
